Archive of Biomedical Science and Engineering
Microwave Irradiated and Conventional Synthesis, Antibacterial Activity Evaluation Studies of Tryptamine-Azole-Fluoroquinolone Conjugates
Yıldız Uygun Cebeci1, Serap Basoglu Özdemir1, Ahmet Demirbas1, Neslihan Demirbas1* and Sengül Alpay Karaoglu2
Cite this as
Cebeci YU, Özdemir SB, Demirbas A, Demirbas 1N, Karaoglu SA (2018) Microwave Irradiated and Conventional Synthesis, Antibacterial Activity Evaluation Studies of Tryptamine-Azole-Fluoroquinolone Conjugates. Biomed Sci Eng 4(1): 010-020. DOI: 10.17352/abse.000009Tryptamine was converted to the corresponding 1,2,4-triazole, 1,3,4-oxadiazole, 5-oxo-1,3-thia(oxa)zolidine and 5-(4-chlorophenyl)-1,3-thia(oxa)zole derivatives via several steps. 1,3,4-oxadiazole and 1,2,4-triazoles were then converted to the corresponding Mannich bases containing fluroquinolone core using a one-pot three-components procedure. Conventional and microwave-assisted methods were applied for all syntheses. All the newly synthesized compounds were screened for their antibacterial and most of them were found to have good–moderate antibacterial activity.
Introduction
The increasing community- and hospital- acquired infectious diseases caused by resistant bacteria to most classes of antibacterial drugs resulted in a pressing and urgent need for designing of new antibiotic candidates. The declaration of The European Centre for Disease Prevention and Control (ECDC) reporting “Every year, the infections caused by resistant bacteria gives rise to 25,000 deaths with a cost of over 1.5 billion Euro because of healthcare spending and labor losses in the Europa” reveals that this is a public health problem with also socio-economic loses [1-5].
In recent years, to overcome the drug resistance problem, the concept of hybrid molecules, which contain two or more pharmacophore groups binding together covalently in one molecular framework, has been introduced in the medicinal chemistry field. These compounds that are obtained by molecular hybridization of several pharmacophore groups, act by inhibiting two or more conventional targets simultaneously, and this multiple target strategy has resulted in the development of a number of bioactive hybrid molecules with desired pharmacokinetic profile, therapeutic index and more importantly less tendency to resistance [6-11].
Heterocyclic compounds accepted as medicinally important products have given a new direction to new drug design and discovery studies. 1,2,4-Triazole, 1,3,4-oxadiazole and 1,3-thiazole derivatives which attract great attention by synthetic and medicinal chemists due to their distinct structures have been regarded as useful tools for drug discovery processes [12-14]. The compounds containing these heterocyclic units in their structures have been reported to possess a wide range of biological activities, such as antibacterial, antitubercular, antiviral, analgesic, antioxidant, anticancer etc [15-18]. Another class of bioactive compounds, indoles exhibit several biological activities with high bioavailability and relatively low toxicity. Indole unit constitutes a part of several natural compounds isolated from marine creatures, medicinal plants or microorganisms. Although a number of studies devoted on the modifications of indole unit was reported, there are only a few studies about indole-azole hybrids. Based on the pharmacodynamic principle of superposition, it is accepted that the indole hybrids containing 1,3,4-oxadiazole and 1,2,4-triazole units at the position 3 exhibit efficient antibacterial activity [19,20].
In the anti-infective chemotherapy field, fluoroquinolones which target two type II bacterial topoisomerase enzymes, DNA gyrase and/or topoisomerase IV, constitute a large and constantly expanding group of synthetic antibiotics. They have been attracting major interest due to broad-spectrum of activity towards both Gram (-) and Gram (+) bacteria by inhibiting bacterial DNA replication, and their chemotherapeutic efficacy [8-11]. In the concept containing the synthesis of new analogs or modifying existing drug compounds, new fluoroquinolones including large substituent at the position 7 were reported by several research groups [7,21-25]. Microwave assisted techniques were reported to be more effective in terms of environment, reaction time, high yields, ease of work-up and isolation of products. Moreover, the environmentally polluting solvents with high boiling points which are often expensive, toxic, difficult to remove are not necessary most of the microwave assisted synthesis [26].
Recently, multicomponent reactions (MCRs) have received considerable attention by synthetic organic and medicinal chemists for the construction of complex molecules having biological activity. When compared with conventional organic reactions and maximal structural complexity MCRs have some superior properties including high conversion rate, minimal reaction time and structural complexity. Thus, MCRs are also considered as green chemical processes [27]. Among these, Mannich reaction, a one pot three-component condensation reaction, provide synthetically and biologically important β-aminoalkylated compounds, which are important intermediates for the construction of various nitrogen-containing natural products and pharmaceuticals [28].
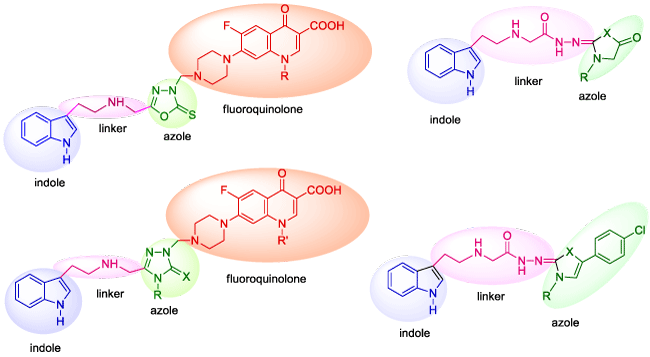
Thus, based on the aforementioned statements, this study focused on the design, ecofriendly synthesis, and antibacterial activity evaluation of new indole-azole-fluoroquinolone hybrids based on pharmacophores hybridization. Indole scaffold was selected as the key prototype structural unit and the integration of indole skeleton, azole and fluoroquinolone pharmacophores we attempted to conventional and MW mediated green synthesizes of new indole-azole hybrid scaffold in the one molecular frame was performed as shown in figure 1 with the aim to prepare new antibacterial agents with preferably therapeutic profile having less tendency to antibacterial resistance.
Results and Discussion
Chemistry
In this study, we attempted to conventional and MW mediated green synthesize of new indole-azole-fluoroquinolone hybrids as possible drug candidates with antibacterial activity. On the basis of 1H, 13C NMR, FT IR and EI-MS data, the structure of the target products was established. All synthesized compounds were checked for purity and identity using elemental analysis. The MICs against clinically important Gram-negative and Gram positive pathogens were determined as well. The synthetic methodologies adopted to obtain the target compounds were depicted in Scheme 1 and Scheme 2.
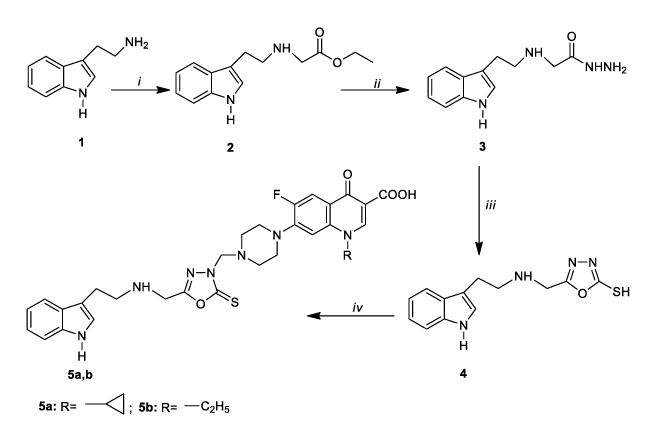
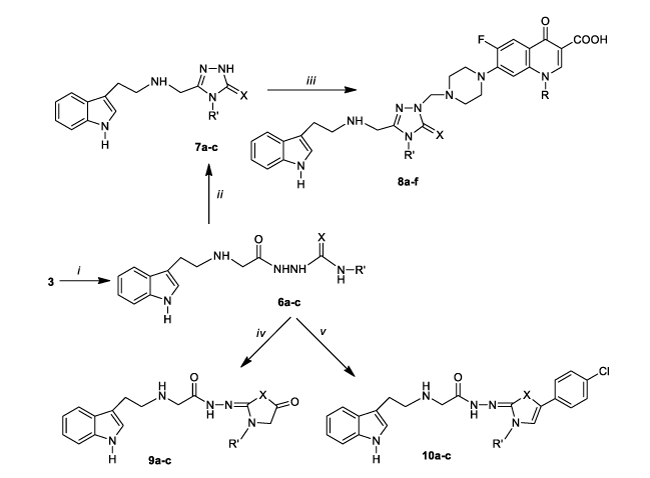
The treatment of the starting compound, tryptamine [2-(1H-indol-3-yl)ethanamine], which was selected considering its biological importance [29-33], with ethyl bromoacetate to give ethyl 2-[2-(1H-indol-3-yl)ethylamino]acetate (2) was carried out under conventional and also microwave (MW) irradiated conditions with a view to maximizing the yield of the product and minimizing the reaction time. With the assessing of MW irradiated method, the yield of the reaction was improved to good level (87 %) however, more significantly, the reaction time for complete consumption of starting materials was lowered from 18 h with conventional stirring to a remarkable 10 min. Moreover, MW irradiated method with no solvent supplied more ecofriendly way. The structure compound 2 was confirmed by the disappearance of broad singlet for NH2 and the presence of triplet at the region 1.90-1.93 ppm and a quartet at 2.85-2.89 ppm due to the presence of ethyl group protons in the 1H NMR spectrum of compound 2. This group appeared at 14.68 ppm (CH3) and 61.18 ppm (CH2) in the 13C NMR spectrum. Further, in the FT IR, the appearance of the stretching band due to C=O group at the region 1619 cm-1 confirmed the formation of ester (2). The substitution of ester group by hydrazide generated 2-[2-(1H-indol-3-yl)ethylamino]acetohydrazide (3), which were confirmed by the appearance of broad signal for -NHNH2 group in FT IR. Further, the protons of hydrazide function appeared at 3.93 (NH2) and 10.94 ppm (NH) as D2O exchangeable singlets confirming the formation of hydrazide. In order to optimize microwave (MW) irradiation conditions, MW was applied at different power values of 120 and 150 W without any solvent, while the conventional synthesis of compound 3 required ethanol as reaction solvent. The complete conversion of the compound 2 in best yield was observed after microwave irradiation at 220 W maximum power for 6 min. Higher MW power or longer reaction time caused to lower yields. Compound 3 gave the corresponding 1,3,4-oxadiazole derivative (4) upon the treatment with carbon disulfide in basic media. The 13C NMR observations revealed the appearance 1,3,4-oxadiazole C-2 and C-5 carbons at the region 166.19 ppm and 163.99 ppm confirming the formation of 1,3,4-oxadiazole [34, 35]. The stretching band characteristic for SH absorption was seen at 2921 cm-1 in the FT IR spectrum. The signal observed at 13.69 ppm in the 1H NMR spectrum was attributed the SH proton. On the other hand, the treatment of compound 3 with alkyliso(thio)cyanates produced the corresponding hydrazincarbo(thio)amides (6a-c) with both conventional and MW irradiated method. In the conventional method, the reaction yielding compounds 6a-c completed in 24 h in DCM with 86-91 % yield, while reaction time was 8 min with the yield 97-99 %. The structures of hydrazinecarbo(thio)amides were confirmed by the presence of additional signals at the related chemical shift values originated from alkyliso(thio)cyanate moiety. This compound exhibited mass fragmental and elemental analysis data confirming the assigned structures.
The basic treatment of compounds 6a-c produced the corresponding 1,2,4-triazoles (7a-c), which can be considered as important tools for further condensation reactions leading to the formation of new bioactive molecules. The reaction was carried out in water-ethanol as a none toxic ecofriendly solvent mixture under reflux and also microwave conditions. With the use of MW conditions, higher yields and lower reaction times were assessed. Microwave irradiation decreased the reaction time from 6 h to 18 min and increased the yields from 56-63 % to 93–97 %. The optimum reaction condition was assessed at 200 W maximum power (Table 1).
The FT IR spectra of compounds 7a-c have -C=S or C=O and -NH stretching bands at 1223-1284 cm-1, 1683 cm-1 and 3163-3397 cm-1, respectively. In the 1H NMR spectra, resonances assigned to the -NH proton on 1,2,4-triazole ring were detected at 10.34-11.40 ppm (NH) which are supported by the literature findings [36-40]. 13C NMR spectra of these compounds have resonances of triazole C-5 and C-3 at 156.63-181.79 ppm and 153.21-171.51 ppm, respectively. The one-pot, three-component Mannich type reaction of compounds 4 and 7a-c with norfloxacin and ciprofloxacin, which are fluoroquinolone class antibiotics, yielded the indole-azole-fluoroquinolone hybrids as new drug candidates (5a,b and 8a-f) with best antibacterial activity. This reaction proceeds via the formation of immonium salt which subsequently attacks the N-1 of triazole or oxadiazole N-3 giving rise to the corresponding Mannich bases. In the preliminary experiment, to optimize the conditions for this condensation, the synthesis of compound 8a was selected as model reaction and various reaction parameters including time, solvent and MW power were screened on the model reaction (Table 2). With the aim to provide further improvement for this synthetic approach, the model reaction was also performed in the presence of polar solvents including THF, H2O, EtOH, and DMF, however, the best result was assessed in solvent free media and the corresponding product was obtained in nearly quantitative yields within 10 min (Table 2, entry 10) in model reaction under microwave irradiation. Secondly, the effect of different catalyst on the reaction yield was screened (Table 2). For this purpose, several Bronsted and Lewis acids including p-TSA, FeCl3, InCl3, HCl were examined in the optimized reaction conditions (Table 2, entry 10) and completion of the reaction was monitored by TLC. The screening studies exhibited that the character of solvent and catalyst has no important influence on the reaction yield. Even so, quite good yields with 83% were obtained with InCl3 (Table 2, entry 14). Furthermore, the TLC analysis showed that the duration of the reaction with catalyst was shorter 3 minutes than the non-catalytic reaction (Table 2, entry 14).
In comparison with the long refluxing time in hazardous solvent, microwave irradiation provided more efficient and green way for one pot Mannich type condensation with relatively higher product yield. The number of signals and their chemical shifts are in accordance with the assigned structures for the Mannich bases. In the 1H and 13C NMR spectra, additional signals corresponding to norfloxacin or ciprofloxacin skeleton were recorded at the related chemical shift values, while the spectra of these compounds showed the disappearance of the characteristic bands of triazole (or oxadiazole)-NH. Moreover, the preparation of Mannich bases was verified by registration of their mass spectrums which were in accordance with their molecular masses and the elemental analysis data (carbon, hydrogen and nitrogen) were ±0.4% of the theoretical values.
Until today, several methods have been developed for the preparation of 1,3-thiazoles, however, Hantzsch synthesis containing the reaction of β-halogenocarbonyl components with the compounds including a thioamide function is the procedure most often referred to. According to the accepted mechanism, the attack of sulfur atom of thioamide, which is present in its ene- thiole form to the halogen atom of carbonyl compound, and this attack is followed by HBr and H2O elimination, which leads to the 1,3thiazole ring formation [36].
As different from the classical Hantzsch reaction, the fact that hydrazinecarbo(thio)amides synthesized in the present study (6a-c) have more than one nucleophilic center and 2-bromo-1-(4-chlorophenyl)ethanone, that is the carbonyl component in this reaction has two position for nucleophilic attacks, can cause the formation of different structural isomers; each of them can exist as their individual E and Z geometrical [3,4,37-40].
In our previous studies [37,38,40], to identify the exact structure of type of compounds 10a-c, full geometric optimization of the possible isomer products was obtained by DFT/B3LYP (density functional theory with B3LYP-the hybrid Becke’s three parameter functional and Lee–Yang–Parr exchange–correlation potential) method with the 6-31G (d,p) basis set and the structure of the molecules was also investigated in detail. According to the obtained results [37,38], the most stable product is Z geometrical isomer with the calculated relative energy 0.000 kcal mol-1. Therefore, thermodynamically, the formation of Z isomers is more favorable. Similar case is present for compounds 9a-c.
Antibacterial activity
All newly synthesized compounds were screened for their antibacterial activity and the results obtained were submitted in Table 3. Most of the compounds synthesized in the present study exhibited activity on the test microorganisms. Among them, 5a,b and 8a-f and which contain a fluoroquinolone nucleus in their structures, demonstrated excellent activities on Gram positive and Gram negative bacteria of the test microorganisms with the mic values <0.24 μg/mL. Compound 4, 6c and 7c demonstrated activity against Mycobacterium smegmatis(Ms), an atipical tuberculosis factor, with the mic values 62.5 μg/mL. No important activity was observed for the remaining compounds. According to the results presented in table 3, it can be concluded that the superior antibacterial activity of compounds 5a,b and 8a-f is due to the presence of fluoroquinolone core in their structures.
Conclusion
This study reports the conventional and successfully developed microwave assisted synthesis of some new hybrid molecules containing several heterocyclic moieties having importance for biological activity. Hence, we combined all these potential chemotherapeutic units, namely indole, 1,3,4-oxadiazole, 1,2,4-triazole, fluoroquinolone and/or 1,3-thiazole moieties.
The structures of new compounds were confirmed by IR, 1H-, 13C NMR, mass spectroscopic and elemental analysis techniques. In addition, the newly synthesized compounds were screened for their antimicrobial activities. The antimicrobial screening suggests that the compounds containing fluoroquinolone core exhibited excellent activities against most of the test microorganisms.
Experimental Section
Chemistry
All the chemicals were purchased from Fluka Chemie AG Buchs (Switzerland) and used without further purification. Melting points of the synthesized compounds were determined in open capillaries on a Büchi B-540 melting point apparatus and are uncorrected. Reactions were monitored by thin-layer chromatography(TLC) on silica gel 60 F254 aluminium sheets. The mobile phase was ethyl acetate/diethyl ether 1:1 and detection was made using UV light. FT-IR spectra were recorded as potassium bromide pellets using a Perkin Elmer 1600 series FTIR spectrometer. 1H NMR and 13C NMR spectra were registered in DMSO-d6 on a Bruker Avance II 400 MHz NMR spectrometer (400.13 MHz for 1H and 100.62 MHz for 13C). The chemical shifts are given in ppm relative to Me4Si as an internal reference; J values are given in Hz. All the compounds gave C, H, and N analyses within ±0.4% of the theoretical values. The mass spectra were obtained on a Quattro EI-MS (70 eV) instrument. Physical parameters for synthetic procedures were given in table 1.
Ethyl 2-(2-(1H-indol-3-yl)ethylamino)acetate (2)
Method 1: To a solution of the corresponding compound 1 (10 mmol) in tetrahydrofuran, triethylamine (20 mmol) and ethyl bromoacetate (10 mmol) were added and the mixture was stirred at room temperature for 24 hours. The precipitate was removed by filtration and the resulting solution was evaporated under reduced pressure to dryness. The crude product obtained was purified by column chromatography (silica gel, hexane/ethyl acetate 7:3).
Method 2: The mixture of ethyl bromoacetate (10 mmol), compound 1 (10 mmol) and triethylamine (20 mmol) was irradiated in monomod microwave reactor in closed vessel with pressure control (Table 1). The crude product obtained was purified by column chromatography (silica gel, hexane/ethyl acetate 7:3).
FT-IR (υ max/cm-1): 3243 (NH), 1732 and 1619 (2C=O), 1117 (C-O). 1H NMR (DMSO-d6, δ ppm): 1.90-1.93 (3H, m, CH3), 2.85-2.89 (4H, m, 2CH2), 3.31 (2H, s, CH2), 3.51 (2H, s, CH2), 6.96-7.09 (3H, m, arH), 7.37 (1H, d, J= 8.0 Hz, arH), 7.53 (1H, d, J= 8.0 Hz, CH), 10.85 (2H, s, 2NH). 13C NMR (DMSO-d6, δ ppm): 14.68 (CH3), 45.48 (CH2), 52.14 (CH2), 61.18 (CH2), 69.90 (CH2), arC: [101.42 (CH), 104.99 (CH), 108.45 (CH), 121.10 (CH), 122.19 (CH), 130.23 (C), 145.75 (C), 154.49 (C)], 172.11 (C=O).EI MS m/z (%): 130.80 (100), 195.88 (68), 152.83 (64), 196.89 (54), 124.80 (43), 162.84 (42), 208.90 (38), 190.94 (37), 210.90 (23), 283.05 ([M+2+K]+, 19).
2-(2-(1H-Indol-3-yl)ethylamino)acetohydrazide (3)
Method 1: A solution of compound 2 (10 mmol) in ethanol was refluxed with hydrazine hydrate (25 mmol) for 15 h. The crude product obtained was purified by column chromatography (silica gel, hexane/ethyl acetate 7:3).
Method 2: The solution of compound 2 (10 mmol) in hydrazine hydrate (25 mmol) was irradiated in a monomod microwave reactor in closed vessel with the pressure control (Table 1). The crude product obtained was purified by column chromatography (silica gel, hexane/ethyl acetate 7:3).
FT-IR (υ max, cm-1): 3259 (NH2+3NH), 3052 (aromatic CH), 1666 (C=O). 1H NMR (DMSO-d6, δ ppm): 2.74 (2H, d, J= 4.0 Hz, CH2), 2.77-2.81 (2H, m, CH2), 3.13 (2H, s, CH2), 3.93 (2H, brs, NH2), 6.94-6.98 (1H, m, arH), 6.99-7.07 (1H, m, arH), 7.12-7.33 (1H, m, arH ), 7.35 (1H, s, arH), 7.51 (1H, d, J= 8.0 Hz, CH), 10.82 (2H, s, 2NH), 10.94 (1H, s, NH). 13C NMR (DMSO-d6, δ ppm): 50.03 (CH2-NH), 51.26 (CH2), 57.97 (CH2), 111.80 (CH), arC: [118.66 (CH), 118.76 (CH), 121.33 (CH), 123.01 (CH), 127.72 (C), 136.70 (C), 169.85 (C)], 170.86 (C=O). EI MS m/z (%): 144.18 (100), 233.29 ([M+1]+, 21), 117.27 (18), 145.31 (12).
5-{[2-(1H-Indol-3-yl)ethylamino]methyl}-1,3,4-oxadiazole-2-thiol (4)
Method 1: CS2 (10 mmol) was added to the solution of compound 3 (10 mmol) in ethanol-water (1:1) and the mixture was refluxed in the presence of KOH (10 mmol) for 8 h. Then, the resulting solution was cooled to room temperature and acidified to pH 4 with 37% HCl. The solid precipitate was collected by filtration and recrystallized from methanol to give the pure compound.
Method 2: The mixture of compound 3 (10 mmol), CS2 (10 mmol) and KOH (10 mmol) in ethanol-water (1:1) was irradiated in monomod microwave reactor in closed vessel with pressure control (Table 1). Then, the resulting solution was cooled to room temperature and acidified to pH 4 with 37% HCl. This was collected by filtration and recrystallized from methanol to give the pure compound. FT-IR (υ max/cm-1): 3247 (NH), 2921 (SH), 1456 (C=N). 1H NMR (DMSO-d6, δ ppm): 1.07 (2H, s, CH2), 3.15 (2H, s, CH2), 395 (2H, s, CH2), 6.99-7.06 (3H, m, arH), 7.08-7.17 (1H, m, arH), 7.34 (1H, d, J=8.0 Hz, CH), 10.85 (1H, s, NH), 10.90 (1H, s, NH), 13.69 (1H, s, SH). 13C NMR (DMSO-d6, δ ppm): 45.32 (CH2), 46.42 (CH2), 50.27 (CH2), arC: [110.43 (C), 112.03 (CH), 118.89 (CH), 121.57 (CH), 123.28 (CH), 123.62 (CH), 12757 (C), 136.71 (C)], 163.99 (oxadiazole C-5), 166.19 (oxadiazole C-2).EI MS m/z (%): 144.31 (100), 117.21 (34), 119.21 (21), 145.31 (12).
General Method for the Synthesis of Compounds 5a, 5b
Method 1: To a solution of corresponding compound 4 (10 mmol) in dimethyl formamide containing InCl3 (1 mmol), ciprofloxacin or norfloxacin (10 mmol) was added and the mixture was stirred at room temperature in the presence of formaldehyde (30 mmol) for 24 h. The solid precipitate was filtered off, washed with water and recrystallized from dimethyl sulfoxide:water (1:1) to give the desired compound.
Method 2: The mixture consisting of compound 4 (10 mmol), ciprofloxacin or norfloxacin, formaldehyde (30 mmol) and InCl3 (1 mmol) was irradiated in monomod microwave reactor in closed vessel with pressure control at 100 W maximum power for 7 min. The obtained solid was washed with water and recrystallized from dimethyl sulfoxide:water (1:1) to give the desired compound.
7-{4-[(5-[(2-(1H-Indol-3-yl)ethylamino)methyl]-2-thioxo-1,3,4-oxadiazol-3(2H)-yl)methyl]piperazin-1-yl}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (5a). M.p. 183-185 oC. FT-IR (υmax, cm-1): 3058 (aromatic CH), 1717 (C=O), 1664 (C=O), 1225 (C=S). 1H NMR (DMSO-d6, δ ppm): 1.13 (2H, s, CH2), 1.27 (2H, s, CH2), 2.73 (4H, s, 2CH2), 2.89 (4H, s, 2CH2), 3.27 (2H, s, CH2), 3.75 (2H, s, CH2), 3.93 (2H, s, CH2), 4.96 (2H, s, CH2), 5.11 (1H, s, CH), 7.03-7.33 (3H, m, arH), 7.45-7.95 (3H, m, arH), 8.61 (2H, s, 2CH), 11.05 (2H, s, 2NH), 15.12 (1H, s, OH). 13C NMR (DMSO-d6, δ ppm): 17.99 (2CH2), 40.61 (2CH2), 49.72 (2CH2), 49.88 (2CH2), 67.71 (2CH2), 106.82 (CH), 107.17 (CH), 111.15 (CH), arC: [107.17 (2CH), 111.15 (CH), 118.63 (CH), 119.49 (CH), 121.87 (CH), 127.93 (C), 137.45 (C), 139.46 (C), 145.49 (C), 148.26 and 150.60 (C, d, JC-F= 234.0 Hz), 152.10 (C), 154.55 (C), 162.78 (C)], 148.10 (quinolone CH), 162.78 (oxadiazole C-2), 167.20 (oxadiazole C-5), 176.67 (C=O). EI MS m/z (%): 675.46 (100), 676.47 (50), 602.40 (36), 618.35 [(M+1), 35]+, 646.39 (19).
7-{4-[(5-[(2-(1H-Indol-3-yl)ethylamino)methyl]-2-thioxo-1,3,4-oxadiazol-3(2H)-yl)methyl]piperazin-1-yl}-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (5b). Mp. 181-183 0C. FT-IR (υmax, cm-1): 3058 (aromatic CH), 1711 (C=O), 1664 (C=O), 1234 (C=S). 1H NMR (DMSO-d6, δ ppm): 1.35 (3H, s, CH3), 2.67 (4H, s, 2CH2), 2.89 (4H, s, 2CH2), 2.27 (2H, s, CH2), 3.95 (2H, s, CH2), 4.53 (4H, s, 2CH2), 4.96 (2H, s, CH2), 7.05-7.18 (3H, m, arH), 7.57-7.60 (2H, m, arH), 7.95 (1H, s, arH), 8.86 (2H, s, 2CH), 11.00 (2H, s, 2NH), 15.30 (1H, s, OH). 13C NMR (DMSO-d6, δ ppm): 14.73 (CH3), 45.20 (CH2), 47.85 (CH2), 49.48 (2CH2), 49.89 (2CH2), 66.90 (2CH2), 70.80 (CH2), 106.30 (CH), 107.51 (C), 111.00 (CH), arC: [111.44 (CH), 111.67 (CH), 118.93 (CH), 119.64 (CH), 121.85 (CH), 122.10 (CH), 128.03 (C), 137.49 (C), 139.10 (C), 140.87 (C), 145.77 and 147.80 (C, d, JC-F= 203.0 Hz), 151.99 (C), 154.47 (C)], 148.79 (quinolone CH), 162.78 (oxadiazole C-5), 163.91 (oxadiazole C-2), 166.55 (C=O), 176.52 (C=O). EI MS m/z (%): 651.38 (100), 663.40 ([M+K+H2O+1]+, 56), 652.38 (40), 576.42 (31), 664.33 (25), 695.37 (19).
General Method for The Synthesis of Compounds 6a-c
Method 1: A mixture of compound 3 (10 mmol) and the corresponding iso(thio)cyanate (10 mmol) in dichloromethane was stirred at room temperature for 24 h. The solid precipitate was collected by filtration and recrystallized from ethanol to afford the desired compound.
Method 2: A mixture of compound 3 (10 mmol) and the corresponding iso(thio)cyanate (10 mmol) in dichloromethane was irradiated in monomod microwave reactor in closed vessel with the pressure control (Table 1). On cooling it to room temperature, a solid appeared. The crude product was recrystallized from acetone:diethyl ether (1:2, v/v) to give the desired product.
2-({[2-(1H-Indol-3-yl)ethyl]amino}acetyl)-N-phenylhydrazinecarboxamide (6a). Mp. 255-256°C. FT-IR (υmax, cm-1): 3345 (5NH), 3044 (aromatic CH), 1770 and 1709 (2C=O). 1H NMR (DMSO-d6, δ ppm): 3.01-3.04 (2H, m, CH2), 3.58-3.68 (2H, m, CH2), 4.17 (2H, s, CH2), 7.03 (2H, d, J= 8.0 Hz, arH), 7.10-7.26 (1H, m, arH), 7.33-7.40 (4H, m, arH), 7.46-7.50 (2H, m, arH), 7.59 (1H, d, J= 4.0 Hz, CH), 10.90 (5H, s, 5NH). 13C NMR (DMSO-d6, δ ppm): 23.72 (CH2), 43.61 (CH2), 50.08 (CH2), arC: [111.42 (C), 111.96 (CH), 118.57 (CH), 118.85 (CH), 121.52 (CH), 123.43 (CH), 127.07 (2CH), 127.60 (C), 128.21 (2CH), 129.19 (CH), 132.76 (C), 136.74 (C)], 155.62 and 169.87 (2C=O). EI MS m/z (%): 339.51 ([M+2]+, 100), 358.47 (84), 358.28 (78), 302.66 (68), 308.98 (62), 359.41 (60), 342.58 (50), 361.41 (34), 378.55 (31), 343.52 (30).
2-({[2-(1H-Indol-3-yl)ethyl]amino}acetyl)-N-phenylhydrazinecarbothioamide (6b). Mp. 148-149°C. FT-IR (υmax, cm-1): 3201 and 2989 (4NH), 1704 (C=O), 1210 (C=S).1H NMR (DMSO-d6, δ ppm): 3.11 (2H, t, J= 16.0 Hz, CH2), 4.04 (2H, t, J= 16.0 Hz, CH2), 4.37 (2H, s, CH2), 7.03-7.27 (2H, m, arH), 7.30-7.32 (3H, m, arH), 7.37 (1H, s, arH), 7.46-7.51 (3H, m, arH), 7.66 (1H, d, J= 4.0 Hz, CH), 10.94 (5H, s, 5NH). 13C NMR (DMSO-d6, δ ppm): 22.80 (CH2), 47.87 (CH2), 53.37 (CH2), 111.08 (C), 112.01 (CH), arC: [118.95 (3CH), 121.61 (2CH), 123.55 (2CH), 127.61 (C), 129.07 (2CH), 134.39 (C), 136.74 (C)], 171.11 (C=O), 181.88 (C=S). EI MS m/z (%): 368.29 (100), 369.36 (30).
N-Benzyl-2-({[2-(1H-indol-3-yl)ethyl]amino}acetyl)hydrazinecarbothioamide (6c). Mp. 148-149°C. FT-IR (υ max/cm-1): 3374 and 3281 (5NH), 3061 (aromatic CH), 1732 (C=O), 1232 (C=S).1H NMR (DMSO-d6, δ ppm): 3.09-3.06 (2H, m, CH2), 4.00 (2H, t, J=8.0 Hz, CH2), 4.28 (2H, s, CH2), 4.79 (2H, d, J=4.0 Hz, CH2), 6.98-7.09 (1H, m, arH), 7.23-7.29 (1H, m, arH), 7.32-7.37 (6H, m, arH), 7.60 (1H, m, arH), 8.68 (1H, brs, CH), 10.05 (2H, s, 2NH), 10.91 (3H, s, 3NH).13C NMR (DMSO-d6, δ ppm): 22.88 (CH2), 44.75 (CH2), 47.00 (CH2), 52.82 (CH2), 111.10 (CH), 127.97 (C), arC: [123.51 (CH), 127.19 (CH), 127.55 (CH), 127.78 (CH), 127.83 (CH), 128.60 (2CH), 128.61 (2CH), 136.81 (C), 139.90 (C), 152.17 (C)], 171.68 (C=O), 181.90 (C=S). EI MS m/z (%): 144.17 (100), 174.27 (40), 214.31 (31), 117.27 (30), 143.17 (28), 359.41 (12), 420.48 ([M+K]+, 11).
General Method for The Synthesis of Compounds 7a-c
Method 1: The solution of the corresponding compound 6a-c (10 mmol) in ethanol:water (1:1) was refluxed in the presence 100 mL of 2 % NaOH for 6 h. Then the resulting solution was cooled to room temperature and acidified to pH 4 with 37% HCl. The precipitate formed was filtered off, washed with water, and recrystallized from ethyl acetate to afford the desired compound.
Method 2: The mixture of the corresponding compound 6a-c (10 mmol) and 2 % NaOH (100 mL) was irradiated in monomod microwave reactor in closed vessel with the pressure control (Table 1). Upon acidification of reaction content to pH 7 with 37% HCl, a white solid appeared. This crude product was filtered off, washed with water and recrystallized from ethyl acetate to afford the desired product.
3-{[(2-(1H-Indol-3-yl)ethyl)amino]methyl}-4-phenyl-1H-1,2,4-triazole-5(4H)-one (7a). Mp. 135-136°C.FT-IR υ max/cm-1): 3397 and 3275 (2NH), 3057 (aromatic CH), 1683 (C=O). 1H NMR (DMSO-d6, δ ppm): 2.99-3.02 (2H, m, CH2), 4.35 (4H, brs, 2CH2), 7.01-7.15 (5H, m, arH), 7.37 (2H, d, J=8.0 Hz, arH), 7.55 (2H, d, J=8.0 Hz, arH), 9.24 (1H, s, CH), 10.91 (2H, s, 2NH), 11.40 (1H, s, NH). 13C NMR (DMSO-d6, δ ppm): 51.41 (CH2), 66.71 (2CH2), 107.02 (CH), arC: [107.27 (CH), 114.68 (CH), 118.73 (2CH), 119.85 (CH), 122.33 (2CH), 129.22 (2CH),135.53 (C), 135.63 (C), 140.07 (C), 152.96 (C)], 155.42 (triazole C-3) ,156.63 (triazole C-5). EI MS m/z (%): 468.34 (100), 446.38 (43), 469.40 (31), 447.51 (17), 353.34 ([M+H2O+2]+, 10).
3-{[(2-(1H-Indol-3-yl)ethyl)amino]methyl}-4-phenyl-1H-1,2,4-triazole-5(4H)-thione (7b). Mp. 135-136°C. FT-IR (υmax/cm-1): 3161 (2NH), 3061 (aromatic CH), 1284 (C=S). 1H NMR (DMSO-d6, δ ppm): 3.07 (2H, t, J= 16.0 Hz, CH2), 4.53 (2H, s, CH2), 4.71-4.73 (2H, d, J=8.0 Hz, CH2), 7.22 (1H, brs, arH), 7.29-7.30 (1H, m, arH), 7.31-7.37 (7H, m, arH), 7.66 (1H, d, J= 4.0 Hz, CH), 8.29 (1H, s, NH), 8.75 (1H, s, NH), 10.90 (1H, s, NH). 13C NMR (DMSO-d6, δ ppm): 46.43 (CH2), 50.29 (CH2), 52.84 (CH2), arC: [112.02 (CH), 115.07 (CH), 118.67 (CH), 118.94 (CH), 121.51 (CH), 123.49 (CH), 123.72 (CH), 127.22 (C), 127.57 (2CH), 128.74 (CH), 136.77 (C), 163.99 (C), 168.63 (C)], 171.51 (triazole C-3), 181.79 (triazole C-5). EI MS m/z (%): 361.41 (100), 364.29 (33), 372.30 ([M+Na]+, 31), 356.28 (20), 332.32 (18).
3-{[(2-(1H-Indol-3-yl)ethyl)amino]methyl}-4-benzyl-1H-1,2,4-triazole-5(4H)-thione (7c). Mp. 135-136°C. FT-IR (υ max/cm-1): 3296 and 3239 (3NH), 3061 (aromatic CH), 1587 (C=N), 1223 (C=S). 1H NMR (DMSO-d6, δ ppm): 2.00 (8H, d, J=8.0 Hz, 4CH2), 7.17 (2H, d, J=8.0 Hz, arH), 7.33 (3H, t, J=8.0 Hz, arH), 7.59 (4H, d, J=8.0 Hz, arH), 9.83 (1H, s, CH), 10.34 (2H, s, 3NH). 13C NMR (DMSO-d6, δ ppm): 18.35 (CH2), 22.27 (CH2), 23.68 (CH2), 25.51 (CH2), 128.48 (CH), arC: [118.26 (2C), 129.08 (CH), 129.15 (CH), 129.25 (CH), 129.29 (CH), 129.40 (CH), 129.86 (CH), 130.00 (CH), 130.45 (CH), 130.53 (CH), 139.53 (2C)], 153.21 (triazole C-3), 176.82 (triazole C-5). EI MS m/z (%): 360.47 (100), 368.42 (49), 361.54 (36), 381.50 (34), 370.42 (13).
General Method for The Synthesis of Compounds 8a-f
Method 1: To a solution of corresponding compound 7 (10 mmol) in dimethyl formamide ciprofloxacin (for 8a, 8c, 8e) or norfloxacin (for 8b, 8c, 8f) (10 mmol) was added and the mixture was stirred at room temperature in the presence of formaldehyde (30 mmol) and InCl3 (1 mmol) for 24 h. The solid precipitate was collected by filtration, washed with water and recrystallized from dimethylsulfoxide:water (1:1) to give the desired compound.
Method 2: The mixture consisting of the corresponding compound 7 (10 mmol), ciprofloxacin (for 8a, 8c, 8e) or norfloxacin (for 8b, 8c, 8f) (10 mmol), formaldehyde (30 mmol) and InCl3 (1 mmol) was irradiated in monomod microwave reactor in closed vessel with the pressure control with 100 W maximum power for 7 min. The solid obtained was washed with water and recrystallized from ethyl acetate to give the desired compound.
7-{4-[(3-[(2-(1H-Indol-3-yl)ethylamino)methyl]-5-oxo-4-phenyl-4,5-dihydro-1,2,4-triazol-1-yl)methyl]piperazin-1-yl}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8a). Mp. 183-185°C. FT-IR (υmax, cm-1): 3269 (NH), 3063 (aromatic CH), 1719 (C=O), 1664 (C=O), 1599 (C=N). 1H NMR (DMSO-d6, δ ppm): 1.18 (2H, s, CH2), 1.31 (2H, s, CH2), 2.09 (2H, s, CH2), 2.70 (2H, s, CH2), 2.73 (2H, s, CH2), 2.89 (2H, s, CH2), 3.71 (2H, s, CH2), 3.82 (2H, s, CH2), 4.58-4.62 (2H, m, CH2), 4.91 (2H, s, CH2), 6.97 (2H, s, arH), 7.25-7.29 (4H, m, arH), 7.48-7.62 (4H, m, arH), 7.89 (1H, s, arH), 8.66 (3H, d, J=4.0 Hz, 3CH), 9.34 (1H, s, NH), 10.60 (1H, s, NH), 15.21 (1H, s, OH).13C NMR (DMSO-d6, δ ppm): 8.02 (CH2), 43.96 (CH2), 48.39 (CH2), 49.80 (CH2), 49.96 (CH2), 50.20 (CH2), 63.05 (CH2), 72.10 (CH2), 75.17 (CH2), 82.41 (CH2), 119.89 (2CH), 120.12 (CH), arC: [120.22 (CH), 120.29 (CH), 120.37 (CH), 120.60 (CH), 120.90 (CH), 123.26 (CH), 128.80 (CH), 128.83 (2CH), 128.88 (2CH), 128.96 (C), 129.13 (C), 139.62 (C), 139.92 (2C), 140.85 (C), 152.86 and 154.05 (C, d, JC-F= 119.0 Hz), 154.37 (2C)], 154.71 (triazole C-3), 155.43 (triazole C-5), 166.38 (C=O), 176.83(C=O). EI MS m/z (%): 685.56 (100), 685.81 (81), 684.31 (62), 675.52 (48), 699.37 [(M+Na), 25]+, 717.51 [(M+K), 21]+, 676.71 [(M), 15]+.
7-{4-[(3-[(2-(1H-Indol-3-yl)ethylamino)methyl]-5-oxo-4-phenyl-4,5-dihydro-1,2,4-triazol-1-yl)methyl]piperazin-1-yl}-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8b). Mp.178-180°C. FT-IR (υmax, cm-1): 3266 (NH), 3063 (aromatic CH), 1708 (C=O), 1663 (C=O), 1598 (C=N).1H NMR (DMSO-d6, δ ppm): 1.41 (3H, d, J=4.0 Hz, CH3), 2.73 (4H, s, 2CH2), 2.89 (6H, s, 3CH2), 3.75 (4H, s, 2CH2), 4.59 (4H, s, 2CH2), 7.01-7.25 (3H, m, arH), 7.26-7.29 (4H, m, arH), 7.50 (4H, s, arH), 7.89 (2H, s, CH), 8.94 (1H, s, NH), 10.50 (1H, s, NH), 15.35 (1H, s, OH). 13C NMR (DMSO-d6, δ ppm): 14.80 (CH3), 36.24 (CH2), 44.02 (CH2), 61.31(CH2), 63.05 (CH2), 72.11 (CH2), 79.96 (2CH2), 82.41 (2CH2), 107.59 (2CH), arC: [120.14 (CH), 120.29 (CH), 120.37 (CH), 128.79 (2CH), 128.83 (3CH), 128.96 (3CH), 137.63 (2C), 139.57 (2C), 139.73 (2C), 140.85 (2C), 152.10 and 154.37 (C, d, JC-F= 227.0 Hz)], 154.60 (triazole C-3), 155.42 (triazole C-5), 166.56 (C=O), 176.62 (C=O). EI MS m/z (%): 685.56 (100), 686.63 (48), 688.45 ([M+Na]+, 28), 683.49 ([M+H2O]+, 17).
7-{4-[(3-[(2-(1H-Indol-3-yl)ethylamino)methyl]-4-phenyl-5-thioxo-4,5-dihydro-1,2,4-triazol-1-yl)methyl]piperazin-1-yl}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8c). Mp. 188-189 °C, FT-IR (υmax, cm-1): 3262 (OH), 3198 (NH), 3128 (NH), 3053 (aromatic CH), 1717 (C=O), 1663 (C=O), 1545 (C=N). 1H NMR (DMSO-d6, δ ppm): 1.16 (2H, d, J=8.0 Hz, CH2), 1.30 (2H, d, J=8.0 Hz, CH2), 2.72 (2H, s, CH2), 2.88 (2H, s, CH2), 3.04 (2H, s, CH2), 3.46 (2H, s, CH2), 4.62 (2H, s, CH2), 4.75 (2H, s, CH2), 5.16 (2H, s, CH2), 5.37 (4H, s, 2CH2), 7.23 (4H, d, J=8.0 Hz, arH), 7.48- 7.58 (7H, m, arH), 8.62 (1H, s, CH), 8.64 (1H, s, CH), 8.89 (1H, s, CH), 9.27 (1H, s, NH), 9.38 (1H, s, NH), 15.20 (1H, s, OH). 13C NMR (DMSO-d6, δ ppm): 8.03 (CH2), 31.24 (CH2), 36.24 (CH2), 36.28 (CH2), 49.93 (CH2), 50.11 (2CH2), 66.17 (2CH2), 68.77 (2CH2), 106.91 (CH), 111.25 (CH), arC: [111.48 (CH), 117.71 and 117.76 (CH, d, J=5.0 Hz), 118.39 (CH), 118.50 (C), 121.47 (CH), 122.15 (CH), 125.55 (C), 128.44 and 128.83 (CH, d, J=39.0 Hz), 129.11 (CH), 129.94 (2CH), 130.04 (CH), 130.12 (CH), 133.56 (C), 139.59 (C), 140.29 (2C), 147.68 and 150.01 (C, d, JC-F= 233.0 Hz), 162.77 (2C)], 148.36 (quinolone CH), 166.38 (triazole C-3), 166.94 (triazole C-5), 171.57 (C=O), 176.77 (C=O). EI MS m/z (%): 512.20 (100), 693.74 ([M+1]+, 85), 631.85 (73), 378.72 (60), 436.90 (34).
7-{4-[(3-[(2-(1H-Indol-3-yl)ethylamino)methyl]-4-phenyl-5-thioxo-4,5-dihydro-1,2,4-triazol-1-yl)methyl]piperazin-1-yl}-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8d). Mp. 180-181 °C.FT-IR (υmax, cm-1): 3262 (OH), 3198 (NH), 3128 (NH), 3053 (aromatic CH), 1717 (C=O), 1663 (C=O), 1545 (C=N).1H NMR (DMSO-d6, δ ppm): 1.39 (3H, t, J=8.0 Hz, CH3), 2.73 (2H, s, CH2), 2.88 (4H, s, 2CH2), 3.03 (4H, s, 2CH2), 3.34 (2H, s, CH2), 4.55 (4H, t, J=4.0 Hz, 2CH2), 5.16 (2H, s, CH2), 6.93-7.87 (11H, m, arH), 7.89 (1H, s, CH), 7.95 (1H, s, CH), 8.48 (1H, s, NH), 8.93 (1H, s, NH), 15.35 (1H, s, OH).13C NMR (DMSO-d6, δ ppm): 14.76 (CH3), 44.94 (CH2), 49.52 (CH2), 50.03 (CH2), 50.15 (2CH2), 51.07 (2CH2), 68.77 (2CH2), 107.52 (CH), arC: [111.48 (CH), 111.48 (CH), 111.71 and 111.76 (CH, d, J=5.0 Hz), 118.49 (CH), 119.68 (CH), 122.27 and 122.14 (CH, d, J=13.0 Hz), 124.06 (CH), 125.04 (CH), 125.73 (CH), 126.42 (CH), 126.53 (CH), 133.55 (2C), 137.61 (2C), 140.29 (2C), 145.91 (2C), 148.84 and 150.92 (C, d, JC-F= 208 Hz)], 147.67 (quinolone CH), 152.05 (triazole C-3), 166.57 (triazole C-5), 166.92 (C=O), 176.58 (C=O). EI MS m/z (%): 577.26 (100), 430.25 (86), 412.38 (73), 681.20 ([M+1]+, 65), 612.45 (39).
7-{4-[(3-[(2-(1H-Indol-3-yl)ethylamino)methyl]-4-benzyl-5-thioxo-4,5-dihydro-1,2,4-triazol-1-yl)methyl]piperazin-1-yl}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8e). Mp.191-193°C. FT-IR (υmax, cm-1): 3260 (NH), 3070 (aromatic CH), 1718 (C=O), 1666 (C=O). 1H NMR (DMSO-d6, δ ppm): 1.17 (2H, d, J=20.0 Hz, CH2), 1.27-1.32 (2H, m, CH2), 2.75 (4H, s, 2CH2), 2.87 (4H, s, 2CH2), 3.28 (2H, s, CH2), 4.29 (2H, s, CH2), 4.76 (2H, d, J=4.0 Hz, CH2), 4.89 (2H, s, CH2), 4.98 (2H, s, CH2), 6.48 (1H, s, arH), 7.02 (1H, s, arH), 7.22-7.25 (6H, s, arH), 7.30 (3H, d, J=4.0 Hz, arH), 7.95 (1H, s, CH), 8.63 (1H, s, CH), 8.66 (1H, s, CH), 8.98 (1H, s, NH), 11.43 (1H, s, NH), 15.21 (1H, s, OH).13C NMR (DMSO-d6, δ ppm): 8.02 (CH2), 22.62 (CH2), 31.24 (CH2), 36.24 (CH2), 44.73 (CH2), 46.99 (CH2), 47.57 (CH2), 48.37 (CH2), 49.90 (CH2), 51.09 (CH2), 52.87 (CH2), 106.82 (CH), 107.19 (CH), arC: [110.83 (CH), 111.28 (CH), 127.18 (CH), 127.79 (CH), 127.82 (CH), 127.95 (CH), 128.48 (C), 128.57 (CH), 128.79 (2CH), 128.91 (2CH), 134.21 (C), 136.77 (C), 136.69 (C), 143.68 and 145.60 (C, d, JC-F= 192.0 Hz), 169.79 (2C), 166.39 (2C)], 148.41 (quinolone CH), 171.65 (triazole C-3), 176.80 (triazole C-5), 178.49 (C=O), 181.92 (C=O). EI MS m/z (%): 735.53 (100), 715.32 (97), 736.41 (93), 697.61 (82), 715.19 (81), 719.40 (80), 731.45 (78), 706.09 ([M]+, 55), 707.60 ([M+1]+, 44), 729.44 ([M+Na]+, 33), 724.48 ([M+H2O]+, 31).
7-{4-[(3-[(2-(1H-Indol-3-yl)ethylamino)methyl]-4-benzyl-5-thioxo-4,5-dihydro-1,2,4-triazol-1-yl)methyl]piperazin-1-yl}-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (8f). Mp. 189-190°C. F T-IR (υmax, cm-1): 3281 (NH), 3067 (aromatic CH), 1716 (C=O), 1667 (C=O). 1H NMR (DMSO-d6, δ ppm): 1.36-1.44 (3H, m, CH3), 2.51 (2H, s, CH2), 2.69 (2H, s, CH2), 2.73 (2H, s, CH2), 2.89 (2H, s, CH2), 4.30 (2H, s, CH2), 4.58 (2H, s, CH2), 4.75 (2H, d, J=8.0 Hz, CH2), 4.89 (2H, s, CH2), 4.98 (4H, s, 2CH2), 7.18-7.28 (8H, m, arH), 7.30 (1H, d, J=4.0 Hz, arH), 7.89-7.95 (2H, m, arH), 8.91 (1H, s, CH), 8.95 (1H, s, CH), 11.42 (2H, s, NH), 15.35 (1H, s, OH). 13C NMR (DMSO-d6, δ ppm): 14.72 (CH3), 46.98 (CH2), 49.54 (CH2), 49.99 (CH2), 51.08 (CH2), 52.85 (2CH2), 79.95 (2CH2), 80.07 (2CH2), 111.49 (CH), arC: [107.52 and 107.55 (C, d, J=3.0 Hz), 111.72 (CH), 118.90 (CH), 119.41 (CH), 119.66 and 119.74 (C, d, J=8.0 Hz), 127.17 (CH), 127.44 (CH), 127.62 (CH), 127.74 (CH), 127.78 (CH), 127.81 (CH), 127.95 (CH), 128.23 and 128.48 (CH, d, J=25.0 Hz), 162.78 (2C), 148.80 and 150.87 (C, d, JC-F= 207.0 Hz), 171.65 (2C), 176.61 (2C)], 148.92 (quinolone CH), 176.61 (triazole C-3), 178.49 (triazole C-5), 181.92 (2C=O). EI MS m/z (%): 685.56 (100), 703.26 (74), 681.29 (73), 651.29 (68), 663.34 (50), 604.14 (49), 695.35 ([M+1]+, 22).
General Method for The Synthesis of Compounds 9a-c
Method 1: A mixture of compound 6 (10 mmol) and ethyl bromoacetate (10 mmol) in absolute ethanol was refluxed in the presence of dried sodium acetate (50 mmol) for 18 h. The reaction mixture was cooled to room temperature and the salt was separated by filtration. After the solvent was removed under reduced pressure, a solid appeared. This crude product was recrystallized from acetone-water (1:1) to afford the desired product.
Method 2: A mixture of compound 6 (10 mmol) and ethyl bromoacetate (10 mmol) and dried sodium acetate (50 mmol) was irradiated in monomod microwave reactor in closed vessel with pressure control (Table 1). The solid obtained was treated with 50 mL of water, the precipitate was collected by filtration and recrystallized from acetone-water (1:1) to afford the desired product.
2-{[2-(1H-Indol-3-yl)ethyl]amino}-N’-(5-oxo-3-phenyloxazolidin-2-ylidene) acetohydrazide (9a). Mp.152-153°C. FT-IR (υmax, cm-1): 3211 (NH), 3092 (aromatic CH), 1595 (C=N), 1693 (C=O), 1720 (C=O), 1241 (C-O). 1H NMR (DMSO-d6, δ ppm): 2.10 (2H, s, CH2), 3.03 (2H, s, CH2), 3.67 (2H, s, CH2), 4.11 (2H, s, CH2), 6.50-6.72 (2H, m, arH), 7.10-7.28 (3H, m, arH), 7.35-7.45 (4H, m, arH), 8.10 (1H, s, CH), 9.20 (1H, s, NH), 10.10 (2H, s, 2NH). 13C NMR (DMSO-d6, δ ppm): 23.70 (CH2), 43.61 (CH2), 50.08 (2CH2), 111.42 (C), 111.95 (CH), arC: [114.37 (CH), 116.15 (CH), 118.56 (CH), 118.85 (CH), 118.96 (CH), 121.51 (CH), 122.33 (CH), 123.42 (CH), 127.06 (CH), 136.73 (C), 140.14 (C), 155.62 (C), 156.52 (C)], 169.87 (2C=O). EI MS m/z (%): 378.12 (100), 563.78 (83), 496.21 (75), 414.52 ([M+Na]+, 66), 217.52 (36).
2-{[2-(1H-Indol-3-yl)ethyl]amino}-N’-(3-phenyl-5-oxothiazolidin-2-ylidene)acetohydrazide (9b). Mp.134-136°C. FT-IR (υmax, cm-1): 3309 (NH), 3109 (aromatic CH), 1727 (C=O). 1H NMR (DMSO-d6, δ ppm): 1.28 (2H, s, CH2), 1.45 (2H, s, CH2), 4.01 (2H, s, CH2), 3.53 (2H, s, CH2+H2O), 7.30-7.42 (3H, m, arH), 7.43-7.49 (3H, m, arH), 7.51 (3H, s, arH), 10.77 (1H, s, CH), 10.89 (3H, s, 3NH).13C NMR (DMSO-d6, δ ppm): 18.71 (CH2), 24.86 (CH2), 32.55 (CH2), 39.20 (CH2), 111.99 (CH), arC: [118.63 (CH), 123.54 (CH), 124.67 (CH), 128.44 (CH), 128.81 (CH), 129.05 (CH), 129.20 (CH), 129.26 (2CH), 140.11 (C), 141.76 (C), 143.82 (C), 150.77 (C), 161.41 (C)], 172.21 (2C=O). EI MS m/z (%): 427.36 (100), 447.44 (70), 429.49 ([M+Na]+, 67), 425.29 (51), 44 1.37 (48), 433.43 (45), 434.43(28).
2-{[2-(1H-Indol-3-yl)ethyl]amino}-N’-(3-benzyl-5-oxothiazolidin-2-ylidene) acetohydrazide (9c). Mp.132-134°C. FT-IR (υmax, cm-1): 3289 (NH), 3032 (aromatic CH), 1709 (C=O), 1599 (C=N). 1H NMR (DMSO-d6, δ ppm): 3.10 (2H, s, CH2), 4.28 (2H, s, CH2), 4.81 (2H, d, J=12.0 Hz, CH2), 4.91 (2H, s, CH2), 5.14 (2H, s, CH2), 6.97- 7.08 (1H, m, arH), 7.22-7.27 (1H, m, arH), 7.29-7.59 (7H, m, arH), 7.61 (1H, s, CH), 9.52 (2H, s, 2NH), 10.91 (1H, s, NH).13C NMR (DMSO-d6, δ ppm): 25.22 (CH2), 39.76 (CH2), 41.76 (CH2), 45.78 (CH2), 52.60 (CH2), 110.95 (C), 111.97 (CH), arC: [121.56 (CH), 123.50 (CH), 127.77 (CH), 127.95 (CH), 128.04 (CH), 128.09 (CH), 128.70 (CH), 128.73 (CH), 128.82 (CH), 136.35 (C), 136.80 (C), 150.11 (C), 171.69 (C), 172.23 (C)], 181.89 (2C=O). EI MS m/z (%): 360.47 (100), 447.44 (95), 327.31 (65), 323.31 (58), 283.20 (55), 381.43 (51), 355.28 (38).
General Method for the Synthesis of Compounds 10a-c
Method 1: A mixture of the corresponding compound 6 (10 mmol) and 2-bromo-1-(4-chlorophenyl)ethanone (10 mmol) in absolute ethanol was refluxed in the presence of dried sodium acetate (50 mmol) for 17-20 h. The reaction mixture was cooled to room temperature and the salt was separated by filtration. After the solvent was removed under reduced pressure, an oily product obtained. This was extracted with 15 mL of ethyl acetate three times. The organic layer was dried on Na2SO4 and evaporated under reduced pressure. The obtained crude product was recrystallized from acetone: petroleum ether (1:1).
Method 2: A mixture of the corresponding compound 6 (10 mmol), 2-bromo-1-(4-chlorophenyl)ethanone (10 mmol) and dried sodium acetate (50 mmol) in absolute ethanol was irradiated in monomod microwave reactor in closed vessel with pressure control (Table 1). The solid obtained was treated with 50 mL of water, the precipitate was collected by filtration and recrystallized from acetone-petroleum ether (1:1) to afford the desired product.
2-{[2-(1H-Indol-3-yl)ethyl]amino}-N’-[5-(4-chlorophenyl)-3-phenyl-oxazol-2(3H)-ylidene] acetohydrazide (10a). Mp.141-142°C. (Method 2), FT-IR (υmax, cm-1): 3294 (NH), 3092 (aromatic CH), 1595 (C=N), 1698 (C=O), 1241 (C-O). 1H NMR (DMSO-d6, δ ppm): 2.15 (2H, s, CH2), 3.10 (2H, s, CH2), 5.45 (2H, s, CH2), 6.95 (3H, s, arH), 7.25 (5H, s, arH), 7.49 (5H, s, arH), 7.97 (2H, s, 2CH), 8.76 (2H, s, 2NH)., 9.80 (1H, s, NH). 13C NMR (DMSO-d6, δ ppm): 23.70 (CH2), 48.25 (CH2), 66.82 (CH2), 114.34 (2CH), arC: [118.94 (2CH), 122.31 (2CH), 127.06 (2CH), 129.07 (2CH), 129.52 (2CH), 130.16 (3CH), 140.14 (2C), 156.49 (2C), 160.31 (2C), 161.4 (2C)], 169.18 (C=O). EI MS m/z (%): 312.25 (100), 547.36 (78), 296.34 (65), 486.23 ([M+1]+, 51).
2-{[2-(1H-Indol-3-yl)ethyl]amino}-N’-[3-phenyl-5-(4-chlorophenyl)thiazol-2(3H)-ylidene] acetohydrazide (10b). Mp.137-138°C. FT-IR (υmax, cm-1): 3319 (NH), 3049 (aromatic CH), 1712 (C=O). 1H NMR (DMSO-d6, δ ppm): 2.15 (2H, s, CH2), 2.70 (2H, s, CH2), 5.46 (2H, s, CH2), 6.57 (1H, s, arH), 7.15-7.35 (11H, m, arH), 7.65 (1H, s, arH), 10.93 (2H, s, 2CH), 11.10 (1H, s, NH), 11.70 (1H, s, NH), 12.66 (1H, s, NH). 13C NMR (DMSO-d6, δ ppm): 31.15 (CH2), 66.83 (2CH2), 102.26 (CH), 116.77 (CH), arC: [127.88 (CH), 128.85 (CH), 128.79 (CH), 128.92 (CH), 129.14 (CH), 129.52 (2CH), 130.12 (2CH), 130.17 (2CH), 131.42 (2CH), 138.34 (2C), 159.63 (2C), 160.75 (2C), 170.33 (2C)], 192.46 (C=O). EI MS m/z (%): 427.36 (100), 447.44 (70), 452.49 ([M+Na]+, 67), 425.29 (51), 44 1.37 (48), 433.43 (45), 434.43(28).
2-{[2-(1H-Indol-3-yl)ethyl]amino}-N’-[3-benzyl-5-(4-chlorophenyl)thiazol-2(3H)-ylidene] acetohydrazide (10c). Mp.148-150°C. FT-IR (υmax, cm-1): 3217 (NH), 3086 (aromatic CH), 1590 (C=N). 1H NMR (DMSO-d6, δ ppm): 4.37 (2H, d, J=4.0 Hz, CH2), 4.91 (2H, s, CH2), 5.46 (4H, s, 2CH2), 7.19-7.59 (8H, m, arH), 7.64 (5H, d, J=8.0 Hz, arH), 10.92 (1H, s, CH), 12.21 (1H, s, CH), 12.37 (3H, s, 3NH). 13C NMR (DMSO-d6, δ ppm): 44.73 (CH2), 52.85 (CH2), 66.83 (2CH2), 110.95 (C), 111.97 (CH), 118.62 (CH), arC: [118.89 (CH), 121.55 (CH), 127.96 (CH), 128.53 (CH), 128.77 (CH), 128.80 (CH), 128.91 (CH), 129.03 (2CH), 129.52 (2CH), 130.17 (2CH), 136.72 (2C), 140.85 (C), 150.36 (C), 171.68 (C), 181.88 (2C)], 191.45 (C=O). EI MS m/z (%): 612.54 (100), 554.78 ([M+K]+, 65), 502.57 (51), 378.56 (45).
Antibacterial activity assessment
The test microorganisms were obtained from the Hifzissihha Institute of Refik Saydam (Ankara, Turkey) and were as follows: Escherichia coli (E. coli) ATCC35218, Yersinia pseudotuberculosis (Y. pseudotuberculosis) ATCC911, Pseudomonas aeruginosa (P. aeruginosa) ATCC43288, Enterococcus faecalis (E. faecalis) ATCC29212, Staphylococcus aureus (S. aureus) ATCC25923, Bacillus cereus (B. cereus) 709 Roma, Mycobacterium smegmatis (M. smegmatis) ATCC607. All the newly synthesized compounds were weighed and dissolved in dimethyl sulfoxide to prepare extract stock solution of 20.000 microgram/milliliter (μg/mL).
The antibacterial effects of the substances were tested quantitatively in respective broth media by using double microdilution and the minimal inhibition concentration (MIC) values (µg/mL) were determined. The antibacterial and antifungal assays were performed in Mueller-Hinton broth (MH) (Difco, Detroit, MI) at pH.7.3 and buffered Yeast Nitrogen Base (Difco, Detroit, MI) at pH 7.0, respectively. The micro dilution test plates were incubated for 18-24 h at 35 °C. Brain Heart Infusion broth (BHI) (Difco, Detriot, MI) was used for M. smegmatis, and incubated for 48-72 h at 35 °C [41]. Ampicillin (10 μg) and fluconazole (5μg) were used as standard antibacterial and antifungal drugs, respectively. Dimethyl sulfoxide with dilution of 1:10 was used as solvent control. The results obtained were presented in table 3.
The support provided by Scientific and Technological Research Council of Turkey (TUBITAK, Project no: 113Z181) and Karadeniz Technical University, BAP, Turkey (Ref. No. 8623) is greatly appreciated.
- Zidar N1, Tomašič T2, Macut H2, Sirc A2, Brvar M, et al. (2016) New N-phenyl-4,5-dibromopyrrolamides and N-Phenylindolamides as ATPase inhibitors of DNA gyrase. Eur J Med Chem117: 197-211. Link: https://goo.gl/gQi7js
- Sherer BA1, Hull K, Green O, Basarab G, Hauck S, et al. (2011) Pyrrolamide DNA gyrase inhibitors: Optimization of antibacterial activity and efficacy. Bioorg Med Chem Lett 21: 7416–7420. Link: https://goo.gl/DJV8FK
- Demirci S1, Demirbas A, Ulker S, Alpay-Karaoglu S, Demirbas N (2014) Synthesis of Some Heterofunctionalized Penicillanic Acid Derivatives and Investigation of Their Biological Activities. Arch Pharm (Weinheim) 347: 200–220. Link: https://goo.gl/hsMBmb
- Ceylan S1, Bektas H, Bayrak H, Demirbas N, Alpay-Karaoglu S, et al. (2013) Syntheses and Biological Activities of New Hybrid Molecules Containing Different Heterocyclic Moieties. Arch Pharm (Weinheim) 346: 743–756. Link: https://goo.gl/MCVQRP
- Bisacchi GS1, Manchester JI (2015) A new-class antibacterial-almost. Lessons in drug discovery and development: a critical analysis of more than 50 years of efforttoward ATPase inhibitors of DNA gyrase and topoisomerase IV. ACS Infect Dis 1: 4-41. Link: https://goo.gl/8mSYhf
- Panda SS1, Liaqat S2, Girgis AS3, Samir A4, Hall CD, et al. (2015) Novel antibacterial active quinolone–fluoroquinolone conjugates and 2D-QSAR studies. Bioorg Med Chem Lett. 25: 3816–3821. Link: https://goo.gl/JqCdH2
- Wang XD1, Wei W1, Wang PF2, Tang YT3, Deng RC, et al. (2014) Novel 3-arylfuran-2(5H)-one-fluoroquinolone hybrid: Design, synthesis and evaluation as antibacterial agent. Bioorg Med Chem 22: 3620–3628. Link: https://goo.gl/yYzoRp
- Li X1, Zhang YK, Plattner JJ, Mao W, Alley MR, et al. (2013) Synthesis and antibacterial evaluation of a novel tricyclic oxaborole-fused fluoroquinolone. Bioorg Med Chem Lett 23: 963–966. Link: https://goo.gl/ECWjKB
- Sharma PC, Jain A, Yar MS, Pahwa R, Singh J, et al. (2015) Synthesis and antibacterial evaluation of novel analogs of fluoroquinolones annulated with 6-substituted-2-aminobenzothiazoles. Arab J Chem 8: 671–677. Link: https://goo.gl/XiXV9d
- Chugunova E1, Akylbekov N2, Bulatova A2, Gavrilov N2, Voloshina A, et al. (2016) Synthesis and biological evaluation of novel structural hybrids of benzofuroxan derivatives and fluoroquinolones. Eur J Med Chem 116: 165-172. Link: https://goo.gl/DGSn8L
- Huang J1, Wang M1, Wang B2, Wu Z1, Liu M, et al. (2016) Synthesis, antimycobacterial and antibacterial activity of 1-(6-amino-3,5-difluoropyridin-2-yl)fluoroquinolone derivatives containing an oxime functional moiety. Bioorg Med Chem Lett 26: 2262–2267. Link: https://goo.gl/iws4pc
- Gilani SJ1, Khan SA, Siddiqui N (2014) Synthesis and Pharmacological Evaluation of a Novel Series of 2-((2-Aryl thiazol-4-yl)methyl)-5-(alkyl/alkylnitrilethio)-1,3,4-oxadiazole Derivatives as Possible Antifungal Agents. J Heterocycl Chem 51: 1893–1897.
- Shi Z, Zhao Z, Huang M, Fu X (2015) Ultrasound-assisted, one-pot, three-component synthesis and antibacterial activities of novel indole derivatives containing 1,3,4-oxadiazole and 1,2,4-triazole moieties. CR Chimie 18: 1320–1327. Link: https://goo.gl/qxMzaW
- Shelke SH, Mhaske PC, Narkhade S, Bobade VD (2013) Synthesis and Antibacterial Activities of Novel Series of 3-(4-(2-substituted thiazol-4-yl)phenyl)-2-(4-methyl-2-substituted thiazol-5-yl)thiazolidin-4-one Derivatives. J Heterocycl Chem 51: 1151-1156. Link: https://goo.gl/CxtL47
- Pidugu VR1, Yarla NS2, Pedada SR3, Kalle AM4, Satya AK (2016) Design andsynthesis of novel HDAC8 inhibitory 2,5-disubstituted- 1,3,4-oxadiazoles containing glycine and alanine hybrids with anticancer activity. Bioorg Med Chem 24: 5611–5617. Link: https://goo.gl/X9Shpz
- Zhang Y1, Liu XH2, Zhan YZ1, Zhang LY1, Li ZM, et al. (2016) Synthesis and biological activities of novel 5-substituted-1,3,4- oxadiazole Mannich bases and bis-Mannich bases as ketol-acid reductoisomerase inhibitors. Bioorg Med Chem Lett., 2016, 26, 4661–4665. Link: https://goo.gl/bkVJFn
- Li YH1, Zhang B1, Yang HK1, Li Q1, Diao PC, et al. (2017) Design, synthesis, and biological evaluation of novel alkylsulfanyl-1,2,4-triazoles as cis-restricted combretastatin A-4 analogues. Eur J Med Chem 125: 1098-1106. Link: https://goo.gl/bkFdZu
- Xu Z, Guo J, Yang Y, Zhang M, Ba M, et al. (2016) 2,4,5-Trisubstituted thiazole derivatives as HIV-1 NNRTI seffective on both wild-typeandmutant HIV-1 reversetranscriptase: Optimization of the substitution of positions 4 and 5. Eur J Med Chem 123: 309-316. Link: https://goo.gl/jCcHx7
- Johansson H, Jørgensen TB, Gloriam DE, Bräuner-Osbornea H, Pedersen DS (2013) 3-Substituted 2-phenyl-indoles: privileged structures for medicinal chemistry. RSC Adv 3: 945–960. Link: https://goo.gl/pKimE5
- Hassam M, Basson AE, Liotta DC, Morris L, Otterlo WAL, et al. (2012) Novel Cyclopropyl-Indole Derivatives as HIV Non-Nucleoside Reverse Transcriptase Inhibitors. Med Chem Lett 3: 470–475. Link: https://goo.gl/zNHhKE
- Kant R1, Singh V1, Nath G2, Awasthi SK3, Agarwal A (2016) Design, synthesis and biological evaluation of ciprofloxacin tethered bis-1,2,3-triazole conjugates as potent antibacterial agents". Eur J Med Chem 124: 218-228. Link: https://goo.gl/YYwMx7
- Xiao ZP1, Wang XD2, Wang PF3, Zhou Y2, Zhang JW, et al. (2014) Design, synthesis, and evaluation of novel fluoroquinolone-flavonoid hybrids as potent antibiotics against drug-resistant microorganisms". Eur J Med Chem 80: 92-100. Link: https://goo.gl/bPNnED
- Karoli T1, Mamidyala SK, Zuegg J, Fry SR, Tee EH, et al. (2012) Structure aided design of chimeric antibiotics, Bioorg Med Chem Lett 22: 2428–2433. Link: https://goo.gl/yQd8zY
- Gordeev MF1, Hackbarth C, Barbachyn MR, Banitt LS, Gage JR, et al. (2003) Novel oxazolidinone–quinolone hybrid antimicrobials". Bioorg Med Chem Lett 13: 4213-4216. Link: https://goo.gl/aCyuXH
- Mentese MY1, Bayrak H, Uygun Y, Mermer A, Ulker S, et al. (2013) Microwave assisted synthesis of some hybrid molecules derived from norfloxacin and investigation of their biological activities. Eur J Med Chem 67: 230-242. Link: https://goo.gl/AmnvcQ
- Sivakumar KK1, Rajasekaran A2, Senthilkumar P2, Wattamwar PP (2014) Conventional and microwave assisted synthesis of pyrazolone Mannich bases possessing anti-inflammatory, analgesic, ulcerogenic effect and antibacterial properties. Bioorg Med Chem Lett 24: 2940–2944. Link: https://goo.gl/NaqTBV
- Fiorot RG, Filho JFA, Pereira TMC, Lacerda VR, Santos B, et al. (2014) A simple and convenient method for synthesis of new amino naphthoquinones derived from lawsoneby catalytic multicomponent Mannich reaction. Tetrahedron Lett 55: 4373–4377. Link: https://goo.gl/BzQ7VH
- Mansoor SS, Aswin K, Logaiya K, Sudhan SPN (2015) An efficient synthesis of β-amino ketone compounds throughone-pot three-component Mannich-type reactions using bismuth nitrate as catalyst. J Saudi Chem Soc 19: 379–386. Link: https://goo.gl/FMgTTr
- El-Sayed MT1, Suzen S2, Altanlar N3, Ohlsen K4, Hilgeroth A (2016) Discovery of bisindolyl-substituted cycloalkane-anellatedindoles as novel class of antibacterial agents against S. aureusand MRSA. Bioorg Med Chem Lett 26: 218–221. Link: https://goo.gl/HjmQkW
- Li MC1, Sun WS1, Cheng W1, Liu D1, Liang H, et al. (2016) Four new minor brominated indole related alkaloids with antibacterial activities from Laurenciasimilis. Bioorg Med Chem Lett 26: 3590–3593. Link: https://goo.gl/U1XXPW
- Salikov RF1, Belyy AY2, Khusnutdinova NS3, Vakhitova YV3, Tomilov YV (2015) Synthesis and cytotoxic properties of tryptamine derivatives. Bioorg Med Chem Lett 25: 3597–3600. Link: https://goo.gl/Lw9wtc
- Berger A1, Kostyan MK1, Klose SI2, Gastegger M2, Lorbeer E, et al. (2015) Loganin and secologanin derived tryptamine–iridoid alkaloids from Palicoureacrocea and Palicoureapadifolia (Rubiaceae). Phytochemistry 116: 162–169. Link: https://goo.gl/mXeUct
- Maa GL, Yang GX, Xiong J, Cheng WL, Cheng KJ, et al. (2015) Salicifoxazines A and B, new cytotoxic tetrahydro-1,2-oxazine containing tryptamine-derived alkaloids from theleaves of Chimonanthussalicifolius. TetrahedronLett 56: 4071–4075. Link: https://goo.gl/DUmLtT
- Bayrak H1, Demirbas A, Karaoglu SA, Demirbas N (2009) Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur J Med Chem 44: 1057-1066. Link: https://goo.gl/xc5whz
- Bayrak H, Demirbas A, Demirbas N, Alpay-Karaoglu S (2010) Cyclization of some carbothioamide derivatives containing antipyrine and triazole moieties and investigation of their antimicrobial activities. Eur J Med Chem 45: 4726-4732. Link: https://goo.gl/XK9Xyj
- Amin KM1, Rahman DE, Al-Eryani YA (2008) Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg Med Chem 16: 5377–5888. Link: https://goo.gl/xcHppm
- Yolal M, Basoglu S, Bektas H, Demirci S, Alpay-Karaoglu S (2012) Synthesis of Eperezolid-Like Molecules and Evaluation of Their Antibacterial Activities. Russ J Bioorg Chem 38: 539-549. Link: https://goo.gl/MAUxM5
- Basoglu S, Yolal, M, Demirbas A, Bektas H, Abbasoglu R, et al. (2014) Synthesis of linezolid-like molecules and evaluation of their antibacterial activities. Turk J Chem 36: 37-53. Link: https://goo.gl/NSrBRX
- Bonde CG1, Gaikwad NJ (2014) Synthesis and preliminary evaluation of some pyrazine containing thiazolines and thiazolidinones as antibacterial agents. Bioorg Med Chem Lett 12: 2151–2161. Link: https://goo.gl/1ea6tx
- Sahin D, Bayrak H, Demirbas A, Demirbas N, Alpay-Karaoglu S (2012) Design and synthesis of some azole derivatives as potential antibacterial agents. Med Chem Res 21: 4485–4498. Link: https://goo.gl/fbzeax
- Willanova M (1999) National Committee for Clinical Laboratory Standard Methods for determining bactericidal activity of antibacterial agents. AppGuid NCCLS 18–19.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
